Heat Engines
A heat engine is any machine which converts heat into useful work for example, a steam engine or a car engine. Real heat engines are complex and there are many ways of converting heat energy into useful work. We can abstract and generalise the workings of any heat engine into three parts:
The Hot Resevoir - heat energy is created by some process such as combustion of a fuel to provide the heat energy.
The working body - converts the heat energy into work. In real heat engines, the conversion process is never 100% efficient, so the work output is always less than the heat energy supplied. However we frequently idealise and assume reversibility.
The cold resevoir - the energy that cannot be turned into work is dumped and goes to heat up the cold resevoir. In practice, the cold resevoir is usually the atmosphere. We also assume that the temperature of the cold resevoir does not increase, it has an infinite heat capacity.
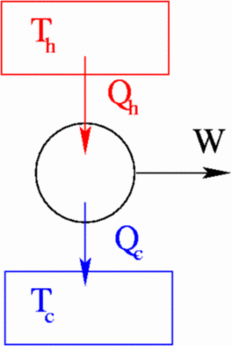
Assume that a heat engine starts with a certain internal energy U, intakes ΔQi heat from a heat source at temperature Ti , does work ΔW , and exhausts heat ΔQf into a the cooler heat reservoir with temperature Tf. With a typical heat engine, we only want to use the heat intake, not the internal energy of the engine, to do work, so ΔU=0. The first law of thermodynamics tells us:
ΔU=0 = ΔQi - ΔQf - ΔW
To determine how effectively an engine turns heat into work, we define the efficiency, η, as the ratio of work done to heat input:
η = ΔW/ΔQi = (ΔQi-ΔQf)/ΔQi
= 1 - ΔQf/ΔQi
Because the engine is doing work, we know that ΔW >0, so we can conclude that ΔQ > 0. Both and are positive, so the efficiency is always between 0 and 1:
Efficiency is usually expressed as a percentage rather than in decimal form. That the efficiency of a heat engine can never be 100% is a consequence of the Second Law of Thermodynamics. If there were a 100% efficient machine, it would be possible to create perpetual motion: a machine could do work upon itself without ever slowing down.
