Latent Heat
When a substance changes phase, that is it goes from either a solid to a liquid or liquid to gas, the energy, it requires energy to do so. The potential energy stored in the interatomics forces between molecules needs to be overcome by the kinetic energy the motion of the particles before the substance can change phase.
If we measure the temperature of the substance which is initially solid as we heat it we produce a graph like Figure 1.
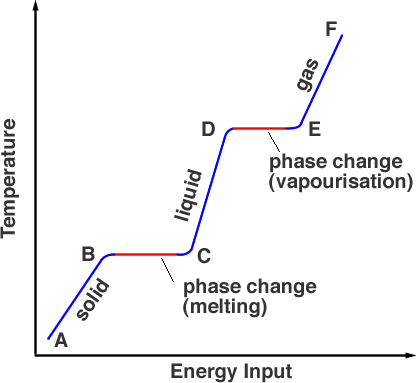
Starting a point A, the substance is in its solid phase, heating it brings the temperature up to its melting point but the material is still a solid at point B. As it is heated further, the energy from the heat source goes into breaking the bonds holding the atoms in place. This takes place from B to C. At point C all of the solid phase has been transformed into the liquid phase. Once again, as energy is added the energy goes into the kinetic energy of the particles raising the temperature, (C to D). At point D the temperature has reached its boiling point but it is still in the liquid phase. From points D to E thermal energy is overcoming the bonds and the particles have enough kinetic energy to escape from the liquid. The substance is entering the gas phase. Beyond E, further heating under pressure can raise the temperature still further is how a pressure cooker works.
Latent Heat of Fusion and Vaporisation
The energy required to change the phase of a substance is known as a latent heat. The word latent means hidden. When the phase change is from solid to liquid we must use the latent heat of fusion, and when the phase change is from liquid to a gas, we must use the latent heat of vaporisation.
The energy require is Q= m L, where m is the mass of the substance and L is the specific latent heat of fusion or vaporisation which measures the heat energy to change 1 kg of a solid into a liquid.
Table 1. show the
| Substance | Specific latent heat of fusion kJ.kg-1 |
°C | Specific
latent heat of vaporisation kJ.kg-1 |
°C |
|---|---|---|---|---|
| Water | 334 | 0 | 2258 | 100 |
| Ethanol | 109 | -114 | 838 | 78 |
| Ethanoic acid | 192 | 17 | 395 | 118 |
| Chloroform | 74 | -64 | 254 | 62 |
| Mercury | 11 | -39 | 294 | 357 |
| Sulphur | 54 | 115 | 1406 | 445 |
| Hydrogen | 60 | -259 | 449 | -253 |
| Oxygen | 14 | -219 | 213 | -183 |
| Nitrogen | 25 | -210 | 199 | -196 |
Heat Pipes
As the density of transitors in a microprocessor increases, the amount of heat disipated increases. A Pentium 4 processor (180 nm running at 2GHz) disipates, 55 Watts of power as heat. Its area is just 131 mm2. This gives a 55 W/(131/(102)) = 42 W cm-2. In comparison a steam iron is 5 Wcm-2.
One solution is the heat pipe. As its name suggests, it transfers heat from high temperature regions to lower temperature regions where there is more space for heat sinks or cooling fans.
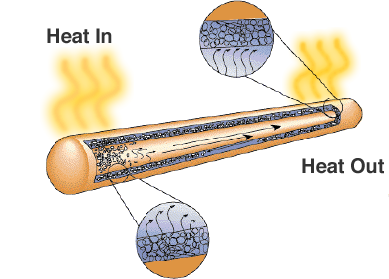
Although it just looks like a sealed metal pipe, there is a wick or porous material and a liquid with a high latent heat of vaporisation. When the pipe is heated the liquid uses the heat to evaporate and changes into a gas, the gas moves to a colder region of the heat pipe where is condenses and uses the latent heat to change back into a liquid. Heat pipes are a reliable and cost effective solution for laptop computers where fans would reduce battery life.
